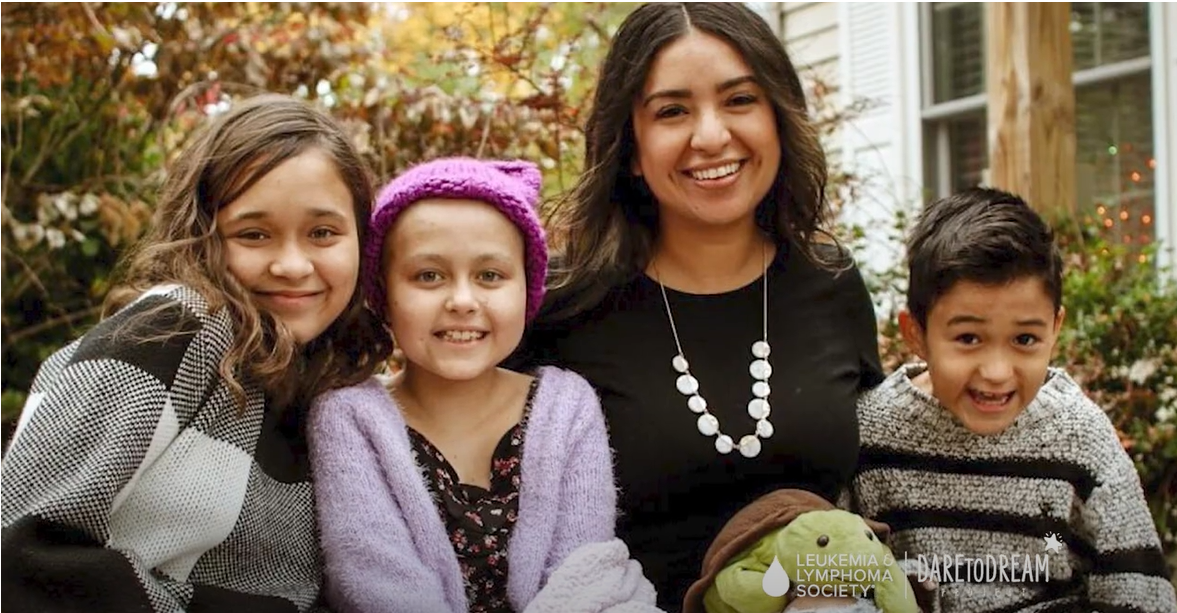Transforming Treatment and Care for Kids with Blood Cancer
Little patients, big impact: Our approach to pediatric oncology
Two pediatric oncologists share their unique background and research approach at AbbVie to help find answers for the youngest patients.
From physician to pharmaceutical leader
As a child in India, Pooja Hingorani dreamt of growing up and becoming a doctor.
As a pediatric oncologist, she made that dream come true and in turn, helped children with cancer so they can make their dreams come true as well.
Hingorani, M.D., who serves as a medical director at AbbVie, was a practicing pediatric oncologist for over 14 years at Phoenix Children’s Hospital and MD Anderson Cancer Center. During her academic career, she had the opportunity to work both in translational and clinical research testing novel therapies in pediatric cancers. This helped her realize the opportunity to make an impact for even more patients by working in the pharmaceutical industry.
Not one-size-fits-all
It’s devasting to hear about a young child with cancer, but the number of cases is relatively low compared to adult diagnosis levels. The reduced number of pediatric patients can mean fewer studies to explore the potential of new treatments. In 2017, the U.S. Congress acted to support more pediatric research and treatment development by passing the RACE Act, which stands for Research to Accelerate Cures and Equity for Children.
Like Hingorani, Gauri Sunkersett, D.O., saw similar areas of opportunity to progress pediatric cancer treatments. She most recently practiced at Johns Hopkins All Children’s Hospital as a pediatric bone marrow and transplantation specialist with a focus on high-risk leukemia and immunotherapy prior to transitioning to join the development team at AbbVie.
“The commitment to advance pediatric research at AbbVie is clear and can be seen in our unique approach to drug development,” shares Sunkersett, an associate medical director for oncology late-stage development. “AbbVie is streamlining the asset development process and connecting early discovery research and development teams with late-stage development teams to identify the potential of an asset early on.”
An early, thorough investigation of potential assets for pediatric application also supports this innovative approach, with attention on differences within the population itself.
“Caring for an infant is not equivalent to treatment for a toddler, much less an adolescent,” Sunkersett says. “Within pediatrics, there are many differences to consider – for example, their disease biology, dosing regimen, and medication administration. It’s not as simple as cutting the adult dose in half.”
Additionally, most pediatric patients are still physically developing while under care. Because a disease can evolve differently in children than in adults, it’s especially important to consider growth and development issues that could arise. Another key element is patients taking their therapy as prescribed and considering which form of medication will be best tolerated for a child.
Taking these considerations into account has supported advancement in research; however, for a percentage of patients who relapse, pediatric clinical trial data is still limited and serves as an opportunity area for the industry, according to Hingorani.
The power of partnerships
In addition to work in the labs and through clinical trials, advancing pediatric cancer care takes a large team that extends beyond the company.
“I don’t think we can make progress unless we come together – no one can do this by themselves,” says Hingorani.
AbbVie partners with The Leukemia and Lymphoma Society (LLS) as part of the Dare to Dream Project, which envisions a world where childhood cancer patients not only survive but thrive after treatment. At the heart of Dare to Dream is LLS PedAL, an international collaborative Pediatric Acute Leukemia (PedAL) Master Trial designed to speed up development of new treatments.
With LLS and additional partners including the National Cancer Institute, part of the National Institutes of Health and the Children’s Oncology Group supporting the PedAL Master Trial, AbbVie is able to extend the reach of research and further investigate treatment options for the youngest patients.
“Through LLS PedAL, the LLS is setting out to fundamentally change how children with pediatric acute leukemia are treated,” said Gwen Nichols, LLS Chief Medical Officer. “We have joined with AbbVie and other international collaborators in a common goal of developing safer, more effective pediatric acute leukemia treatments. Transforming pediatric leukemia cancer care is a group effort and we are thankful for AbbVie’s participation as our first pharmaceutical partner in this groundbreaking endeavor that will help accelerate more treatment options for children.”
To learn more about PedAL, go to http://www.lls.org/dare-to-dream/pedal or call (800) 955-4572 to speak to an Information Resource Specialist.
Media inquiries:
[email protected]