How AbbVie is bringing antiviral expertise to the COVID-19 battle
At the first sign of the pandemic, AbbVie began evaluating ways to help, including accelerating discovery efforts and external collaborations.
Searching for ways to combat COVID-19
Every day for months, Teresa Ng has been receiving pop-up notifications of new research reports related to COVID-19 on her computer.
In a way, this is good. She is getting to know her target better every day.
Ng is a 20-year veteran of AbbVie, where she is a principal research scientist and has helped discover and develop antiviral therapies for both hepatitis C virus (HCV) and HIV. Today, she’s bringing that experience to bear during an unprecedented global pandemic. That’s because Ng, like so many other scientists around the world, is trying to answer the call of a lifetime. She’s part of the AbbVie research team that’s looking for a way to combat COVID-19.
“We’ve built a deep expertise on antiviral therapies, particularly through our work with HCV and HIV, and now we have the opportunity to apply what we’ve learned to one of the most urgent scientific challenges of our lifetime,” Ng said.
It’s a daunting mission, but here’s some good news: As the world faces an unparalleled healthcare crisis in the battle against the COVID-19 pandemic, clinicians, researchers, governments and non-governmental organizations are pooling their resources, combining expertise and sharing preliminary data to help accelerate a race for effective treatments.
While developing an effective vaccine is critical, it is also important to have other options for treatment. People need options for treatment if they become infected with COVID-19. Also, there are a number of people who might not react well to a vaccine, due to their age or a previously weakened immune system.
That’s where antivirals like the one AbbVie is working on come in.
Answers for the disease have still not been found, but a number of promising leads are under investigation. Building on its expertise in antibody drug discovery and virology, AbbVie is collaborating with leading global research institutes to investigate and unlock the potential of fully humanized monoclonal antibodies that may help fight COVID-19.
At the first sign of the pandemic, AbbVie quickly began evaluating possible ways to address the challenge. AbbVie connected with Harbour BioMed, a company that had previously partnered with AbbVie and had recently tested antibodies that they had developed for SARS-CoV (the original SARS virus) for potency against SARS-CoV-2 (the novel virus that causes COVID-19). They found one antibody that could work against both viruses, and published the results in Nature Communications.1 Soon after, Harbour BioMed and two academic institutions, Utrecht University and Erasmus Medical Center, teamed up with AbbVie to develop this monoclonal antibody as a therapy to prevent and treat COVID-19.2 Knowing that there wasn’t a moment to spare, the four entities hit the ground running on their work together the moment they could.
“The world needs an answer for this pandemic ASAP and, instead of starting from scratch, we are collaborating with external experts to quickly develop a treatment,” Ng said.
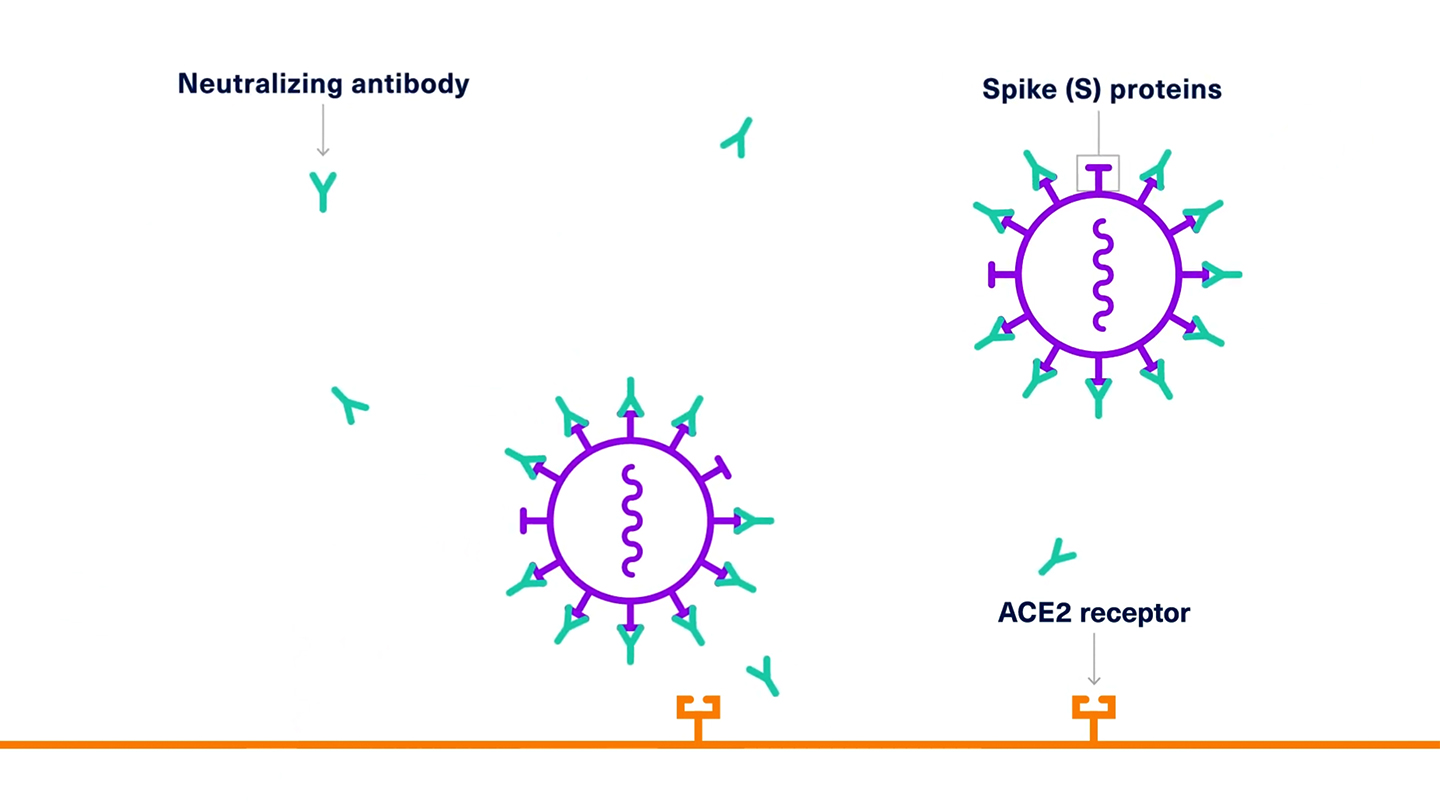
Antibody therapies have received attention for their potential to block SARS-CoV-2 before it can infect a person’s cells. SARS-CoV-2 has a protein on its surface that is involved in recognizing and enabling its entry to the host cells. After the viral entry, the virus injects its genetic material into the host cell, where it hijacks the host’s cellular machinery to make more copies of the virus. One possible way to fight the virus is to discover an antibody that blocks this viral entry protein from interacting with host cells.
To discover the antibody and help speed up development, Harbour BioMed used a type of mouse that has been engineered to produce antibodies that are humanized, which means they are safe and effective for humans. One candidate that has emerged from these efforts has shown the ability to neutralize SARS-CoV-2 in human cells grown in the lab.1
Initial testing was performed at Harbour BioMed in collaboration with Utrecht University and Erasmus Medical Center. AbbVie has helped with further characterization and a fast-track development plan to move this antibody to clinical studies as soon as possible.
"The SARS-CoV-2 pandemic has highlighted the importance of understanding coronavirus biology," said Berend-Jan Bosch, Ph.D., associate professor, research leader at Utrecht University. "The collaboration with AbbVie provides an excellent opportunity to translate our research into a clinical candidate with great potential for advancing the fight against this disease."
There’s more than one possible way to discover an antibody to stop SARS-CoV-2. For example, AbbVie is also looking at the potential for human blood cells to naturally produce the best possible antibodies against SARS-CoV-2.
“When we heard about the pandemic in the early days, we realized that we had an opportunity to work with our existing technology to produce antibodies to help stop the spread of COVID-19,” said Jane Seagal, senior principal research scientist at AbbVie who leads the team that works on internal antibody discovery efforts for SARS-CoV-2 and other coronaviruses using convalescent patient samples, which are samples from patients who have recovered from COVID-19.
“There’s a lot of pressure to find an antibody that will be successful because it’s so urgently needed to help save lives, and as a scientist, I couldn’t imagine a more important application of our expertise.”
An antibody answer to the COVID-19 problem is particularly appealing because of what scientists have learned about SARS-CoV-2. Identification of antibodies that can target both SARS-CoV-2 and potential future pandemic coronaviruses is challenging but might be possible. Many other coronaviruses share a highly similar target region for an antibody to attach. SARS-CoV-2 also mutates much less frequently than other RNA viruses, like the flu virus.
“That’s promising news,” Ng added. “Because this suggests that an antibody treatment might work across different coronaviruses, which means we’re working on possible treatments for COVID-19 and future coronaviruses we don’t even know about yet.”
Tom Hudson, M.D., senior vice president of R&D and chief scientific officer at AbbVie, sees partnerships as key to beating COVID-19, and is encouraged by the speed and urgency with which different organizations have come together.
“The biomedical community needed to work together to find a path to prioritize clinical studies of promising drugs,” he said. “That’s exactly what we hope to accomplish with our partnerships.”
References:
1. Wang, C., Li, W., Drabek, D. et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 11, 2251 (2020). https://doi.org/10.1038/s41467-020-16256-y
2. Press release. AbbVie, Harbour BioMed, Utrecht University and Erasmus Medical Center Announce Collaboration to Develop Monoclonal Antibody Therapy to Prevent and Treat COVID-19 https://news.abbvie.com/2020-06-05-AbbVie-Harbour-BioMed-Utrecht-University-and-Erasmus-Medical-Center-Announce-Collaboration-to-Develop-Monoclonal-Antibody-Therapy-to-Prevent-and-Treat-COVID-19
Media Inquiries:
Email: [email protected]